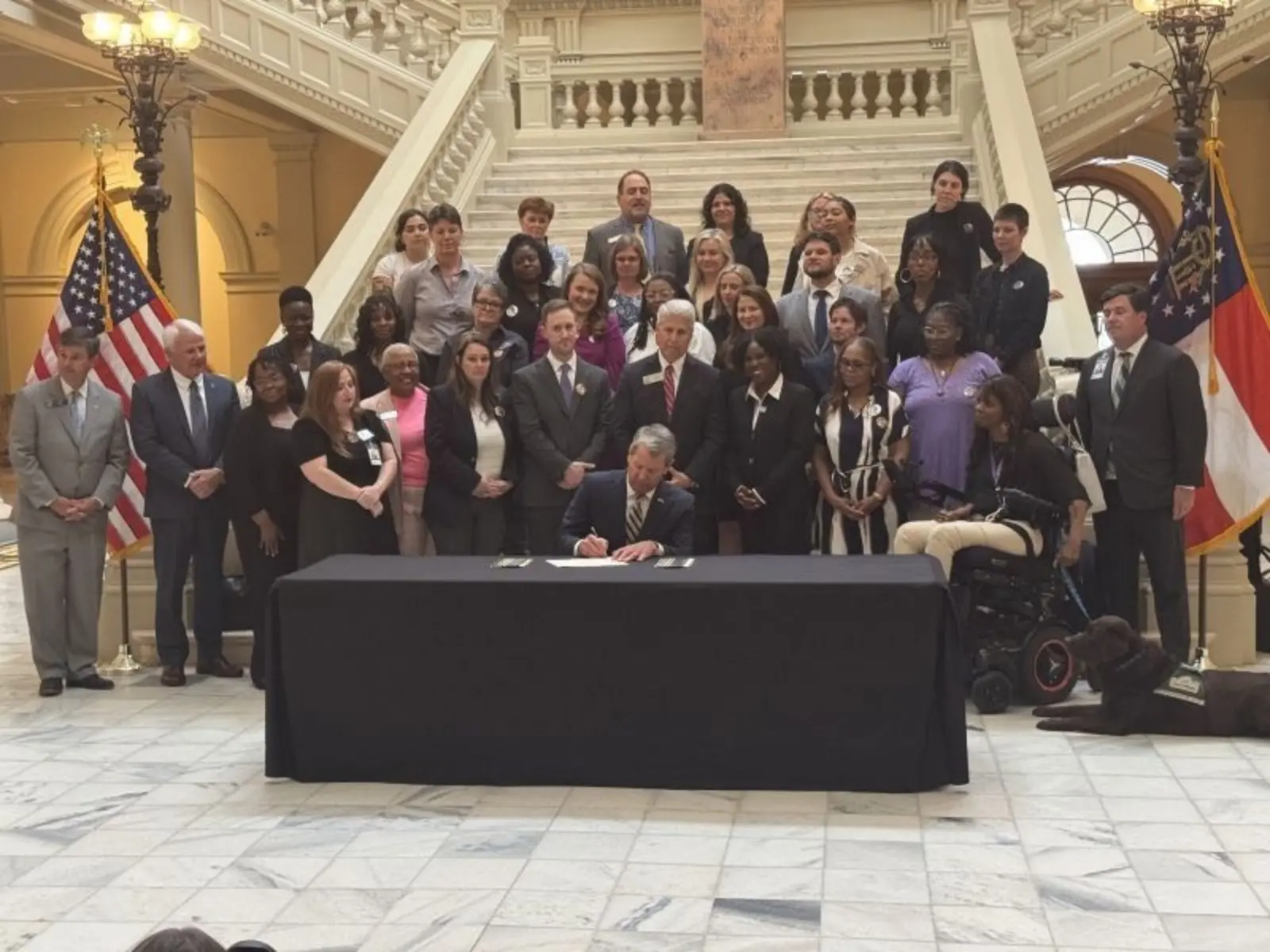Lawmakers Preserve Public Safety While Reducing Long Prison Sentences
Community Safety
Lawmakers Preserve Public Safety While Reducing Long Prison Sentences
Federal Tax Policy
Opportunity Zones: A Tax Break That Missed Its Target
Federal Tax Policy
Taxing the Investment Income of Foundations Is Consistent With Good Tax Principles, but Fixes to OBBB Plan Are in Order
Federal Tax Policy
Impoundments: FY25/FY26 Impacts and Options for Reform
AV’s Scott Hodge: Why Universities Shouldn’t Get Better Tax Treatment Than Retirees
Federal Tax Policy
AV’s Scott Hodge: Why Universities Shouldn’t Get Better Tax Treatment Than Retirees
Is There Pork in the One Big Beautiful Bill? Let Me Count the Ways…
Responsible Tax Reform
Is There Pork in the One Big Beautiful Bill? Let Me Count the Ways…
States Poised to Move Forward in Lowering Drug Costs
Drug Prices
States Poised to Move Forward in Lowering Drug Costs
AV research grantee explores how to bring students back to school
Higher Education
AV research grantee explores how to bring students back to school
“One of the most important and influential voices and actors in philanthropy”
“One of the most important and influential voices and actors in philanthropy”
Webinars offer advice on applying for requests for proposals on policies that work
Evidence and Evaluation
Webinars offer advice on applying for requests for proposals on policies that work
Prescription for Higher Prices: The Real Cost of the EPIC Act
Drug Prices
Prescription for Higher Prices: The Real Cost of the EPIC Act
Maryland Policymakers Promote Public Safety and Second Chances
Community Safety
Maryland Policymakers Promote Public Safety and Second Chances
Commercial Rates in Medicaid: State-Directed Payments Need Scrutiny
Medicaid
Commercial Rates in Medicaid: State-Directed Payments Need Scrutiny
Higher Debt Means Higher Inflation
Federal Tax Policy
Higher Debt Means Higher Inflation
Promote Economic Growth, Not Rent-seeking
Federal Tax Policy
Promote Economic Growth, Not Rent-seeking
New Research Brief Finds Billions in Savings for Seniors
Commercial Sector Prices
New Research Brief Finds Billions in Savings for Seniors
Federal Tax Policy
Imposing an Excise Tax on “Buy-Borrow-Die” Transactions Could Save Up to $147 Billion
A Smart Fix to Boost American Manufacturing and Make Tax Reform More Fiscally Responsible
Federal Tax Policy
A Smart Fix to Boost American Manufacturing and Make Tax Reform More Fiscally Responsible
AV’s Scott Hodge Featured in Chronicle of Philanthropy for Leading the Charge on Nonprofit Tax Reform
Federal Tax Policy
AV’s Scott Hodge Featured in Chronicle of Philanthropy for Leading the Charge on Nonprofit Tax Reform
Why Site-Neutral Payments Matter, Cutting Through Confusion
Same Service, Same Price
Why Site-Neutral Payments Matter, Cutting Through Confusion
Evidence and Evaluation
A second round of requests for proposals to find policies that work
Federal Tax Policy
AV’s Scott Hodge Makes the Case for Reforming the Tax Code for Elite Universities
Federal Tax Policy
A Pro-Growth Approach to Capping the SALT Deduction for Businesses
About Us
We strive to maximize opportunity and minimize injustice by investing in systemic solutions that will outlast our funding.